It can be known from the news that many new energy vehicles that use lithium titanate batteries as the power source in China have already been put into operation. A number of companies such as Beiqi Foton Motor Co., Ltd., Jinlong United Automotive Industry (Suzhou) Co., Ltd., Xiamen Jinlong Automobile Group Co., Ltd., Anhui Ankai Automobile Co., Ltd. and Zhuhai Yinlong New Energy Co., Ltd. have also launched titanium. New energy bus for lithium acid battery.
The power battery is the energy source of the new energy vehicle, and the performance of the power battery determines the performance of the vehicle. Power batteries include lithium ion batteries, nickel metal hydride batteries, super capacitors, fuel cells, and the like. At present, the main type of power battery used in new energy vehicles is a lithium ion power battery.
The lithium ion power battery is composed of four main parts: a positive electrode, a negative electrode, a separator and an electrolyte. The positive electrode of the lithium ion battery is generally a material having a high potential and reversibly deintercalating lithium ions, such as lithium manganate, lithium iron phosphate, ternary materials and the like. The negative electrode of a lithium ion battery is generally a carbon negative electrode material such as graphite. At present, the research on cathode materials has reached a bottleneck, so the research on anode materials has become another key. Lithium titanate is the leader in the anode material, so we talk about the research and development status of lithium titanate in power batteries.
First, lithium titanate properties
The ideal anode material of the power battery should have the following properties: high charge and discharge efficiency and cycle life; high structural stability, chemical stability and thermal stability; high specific capacity, good safety; abundant resources, low price, relative preparation It is simple and does not pollute the environment.
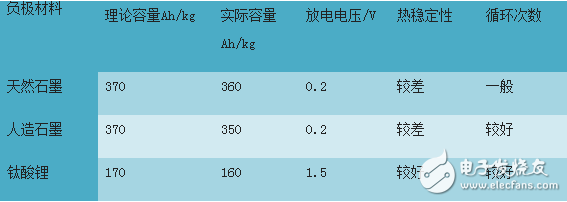
The current research on anode materials for lithium ion batteries is mainly focused on carbon-based materials. The potential of the carbon electrode after lithium insertion in the battery reaction process is close to the potential of the metal lithium. Once the battery is overcharged, the surface of the carbon electrode is likely to precipitate metallic lithium. The generated metal lithium reacts with the electrolyte to generate a flammable gas, which brings considerable safety hazards to the power battery. In addition, the negative electrode of the graphite material has a problem of co-intercalation with the electrolyte, is highly sensitive to the electrolyte, and has limited stability, which causes the cycle stability of the electrode to be affected. The alloy-based anode material has a higher specific capacity than the carbon anode material, and the repeated insertion and removal of lithium during charging and discharging causes a large change in the volume of the alloy-based anode to cause deterioration in cycle performance. Some properties of graphite and lithium titanate anode materials are shown in the table below.
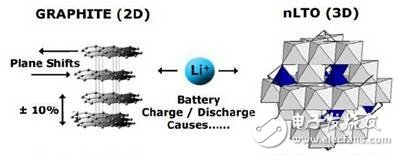
Lithium titanate, Li4TI5O12, face-centered cubic spinel structure. The commonly used compound has the molecular formula AM2O4, the space group is Fd3m, and the unit cell parameter a=0.836 nm. This spinel structure has a certain accommodation space for lithium ions, and a lithium titanate can accommodate three lithium ions. Lithium ion intercalation and deintercalation during charge and discharge have little effect on the structure of the lithium titanate material because the crystal structure of lithium titanate hardly changes, and the value of a increases only from 0.836 nm to 0.837 nm. This phenomenon is called "zero strain", so lithium titanate is also called "zero strain material." This property plays an important role in the electrode material, and can avoid structural changes due to the expansion and contraction of the material during charging and discharging, thereby improving the performance of the electrode and reducing the large attenuation of the specific capacity, thereby prolonging the service life of the battery. .
The chemical diffusion coefficient of lithium titanate (generally 2 & TImes; 10-8 cm2 / s) is larger than the diffusion coefficient of the carbon negative material - an order of magnitude, and the high diffusion coefficient means that lithium titanate is faster than other carbon negative materials. More cycle charge and discharge capacity. However, lithium titanate itself does not provide a lithium source, so it can only be used with lithium-containing electrode materials. When lithium titanate is used as the positive electrode, the negative electrode can only be metallic lithium or lithium alloy. At this time, the voltage of the battery is about 1.5V. Lithium titanate can be used as a negative electrode and a positive electrode material such as LiCoO2 or LiMn2O4 to form a battery having a voltage of 3V or higher. Although lithium titanate can be used as a positive electrode as a positive electrode, since the potential of Li+/Li is 1.5 V, research and application as a positive electrode material are not many. The spinel structure has a three-dimensional lithium ion diffusion channel, so lithium titanate also performs well in high and low temperature performance.
The high potential (1.5 V vs Li+/Li) of lithium titanate means that the SEI film which is usually produced on the surface of the carbon negative electrode in contact with the electrolyte is hardly formed on the surface of the lithium titanate as compared with the carbon negative electrode material. It is also difficult to form lithium dendrites on the surface of lithium titanate in the normal voltage range. This is important because the possibility of lithium dendrites causing a short circuit inside the battery is largely avoided. Therefore, the safety of a lithium ion battery using lithium titanate as a negative electrode is relatively high in various types of lithium ion batteries.
Auto Switches Panels,Marine Switch Pane,Usb Car Charger,Digital Voltmeter Display
Dongguan Andu Electronic Co., Ltd. , https://www.idoconnector.com